
Two new therapies for the treatment of dry eye disease (DED) are being developed by Redwood Pharma. To reduce the effects of dry eye, they are targeted for use at different stages of treatment. RP501 is intended as a first-line treatment of DED in all sufferers, while RP101 will be positioned as a second-line treatment for the largest underserved segment of post-menopausal women with moderate-to-severe DED.

RP501
New first-line treatment of DED based on IntelliGel platform
A next generation smart hydrogel (IntelliGel) product is being developed to help provide temporary relief from DED symptoms. As a first-line therapy, this will help both men and women of all ages with dry eye symptoms that present themselves during the day. Where current products such as artificial tears (saline solutions) may need to be applied many times daily, and where current gels can be difficult to administer and disturb visual acuity, RP501 is intended to be easily applied only once or twice for clinically-proven relief from a wide variety of DED symptoms and lets patients get on with their day.
Applied from a disposable ampoule above the eye, RP501 behaves like water at room temperature. As the drop settles, the natural heat on the surface of the eye causes the RP501 lubricating drop to gel and provide long, sustained relief. This heat-induced hydrogel (thermogel) uses a unique ingredient to increase the amount of water delivered to the eye and reduces the amounts of polymer. RP501 can help stabilize the tear film, reduce inflammation and symptoms of DED. RP501 is non-allergenic and transparent. Categorized as a medical device, RP501 is closer to commercialization and market than a prescription pharmaceutical.
RP101
A prescription, second-line topical chronic DED treatment for post-menopausal women
To address patient needs when artificial tears and other first line therapies fail, the company is developing a second-line, low-dose, estrogen-based topical eye therapy that can help post-menopausal women who suffer from chronic DED. Currently, no sufficiently reliable treatments exist for women with moderate-to-severe symptoms. Furthermore, today’s therapies treat symptoms of DED rather than the root cause of the disease.
The Redwood Pharma product under development will be the first to target the basic biological mechanism related to local estrogen deficiency. As the amount of circulating systemic estrogen can drop by over 90% when women go through menopause, vital tissues dry up and cause problems for patients. Whereas estrogen today is the leading therapy for vaginal atrophy, RP101 will similarly deliver a low-dose of the active substance topically to the eye to help reduce DED symptoms. RP101 has demonstrated safety and efficacy across a wide variety of measures in a European Phase II clinical trial. This study confirmed results from two prior Phase II clinical trials in the US.
Patients will administer a drop of RP101 to the front of the eye, at most twice a day, whereby the IntelliGel drug delivery system will control the release of estrogen. As with RP501, the IntelliGel vehicle behaves like water at room temperature, but forms a gel upon contact with the front of the eye. By remaining on the front of the eye for a prolonged period, estrogen is allowed to act and increase production of tear fluid, thus stabilizing the tear film and reducing symptoms of dry eye.
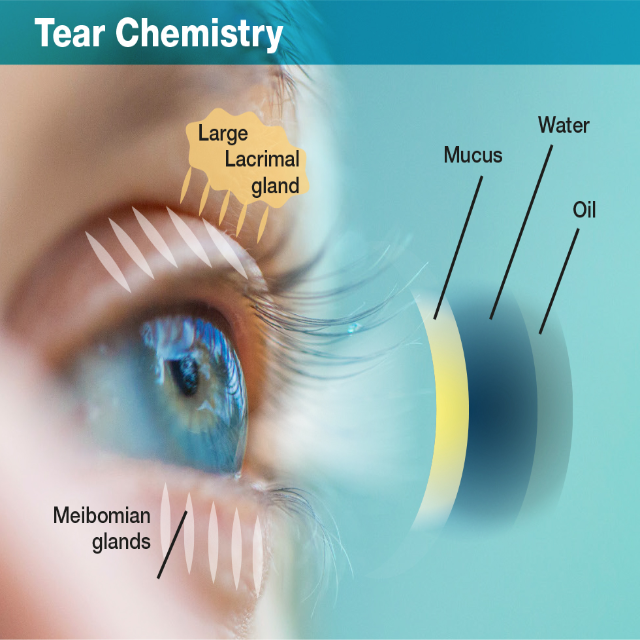
Dry Eye Disease
Sufferers of dry eye experience dryness, pain, stinging, burning, redness, itchiness, blurred vision, mucus discharge and general discomfort in the front of the eye – impairing people in basic daily functions such as reading, operating machinery and driving an automobile. In its severest form, it is even debilitating. If not treated, some cases of dry eye can lead to damage of the ocular surface and even potential blindness.
DED is caused by many factors including inflammation, hormonal changes, dysfunction of glands & cells in and around the eye. Ageing, contact lens use and dry environments can increase the risk of obtaining DED. Patients are forced to used standard over-the-counter eye drops or a combination of other drugs and surgery to try to alleviate their symptoms with the hope of finding any type of effect. There is currently no product on the market today that addresses the root cause of DED.